As we have learnt in school ionic bonds,convent bonds,Hydrogen bond and van der waals bonds are the main chemical bond that exit in nature. Now we have to update that note by adding the new type of chemical bond. It is called as ” Vibrational Bond”.The Chemists of University of British Columbia could confirm the existence of the new bond using the muonium (Mu , A hydrogen isotope) and bromine atoms.
This vibrational bond seems to break the law of chemistry that states if you increase the temperature, the rate of reaction will speed up. In 1989, a team from the University of British Columbia, muonium and bromine reacted and they investigated that the reaction time of , muonium and bromine speed up as the temperature decreased. According to the one of the team, chemist Donald Flemming, the lightweight muonium atom would move rapidly between two heavy bromine atoms, ‘like a Ping Pong ball bouncing between two bowling balls. The oscillating atom would briefly hold the two bromine atoms together and reduce the overall energy, and therefore speed, of the reaction
 |
| Vibrational bonds Photo Credit : Flemming et. al., 2014 |
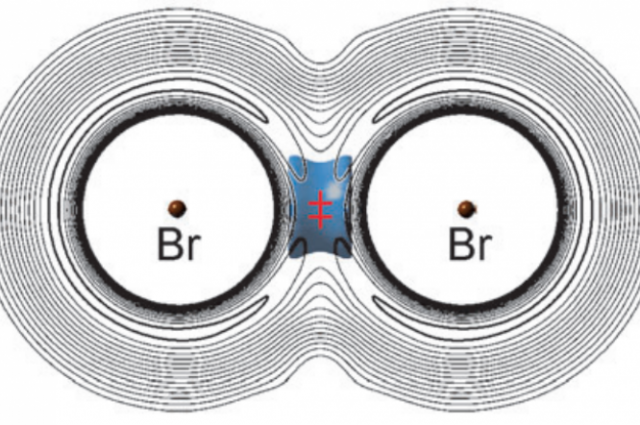
Flemming’s team watched as the light muonium and heavy bromine formed a temporary bond. “The lightest isotopomer, BrMuBr, with Mu the muonium atom, alone exhibits vibrational bonding in accord with its possible observation in a recent experiment on the Mu + Br2 reaction. According to their report, BrMuBr is stabilised at the saddle point of the potential energy surface due to a net decrease in vibrational zero point energy that overcompensates the increase in potential energy
As a summury the vibration in the bond decreased the total energy of the BrMuBr structure, which means that even when the temperature was increased, there was not enough energy to see an increase in the reaction time.
According to their article the vibrational bond confirm and It will be added to the list of chemical bonds.
Photo Credit : Flickr Masakazu Matsumoto
Source : scientific american